G protein-coupled receptors (GPCRs) belong to membrane proteins with extensive hydrophobic
surfaces in the intramembrane region, which are difficult to stabilize in polar aqueous
solutions after dissociation from the membrane and usually cannot use conventional
crystallographic methods to obtain their structural information.
The human glucagon
receptor (GCGR), a member of the class B GPCR family, is essential for glucose homeostasis and
is expected to be a potential drug target for the treatment of diabetes and obesity. In this
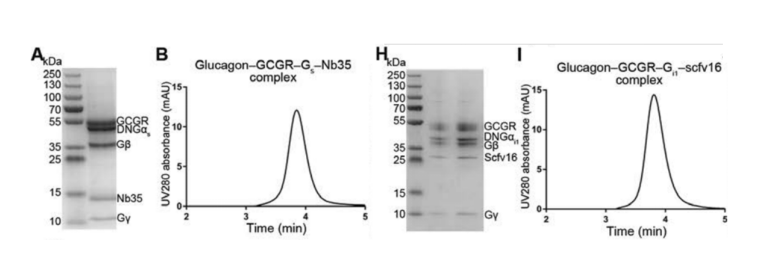
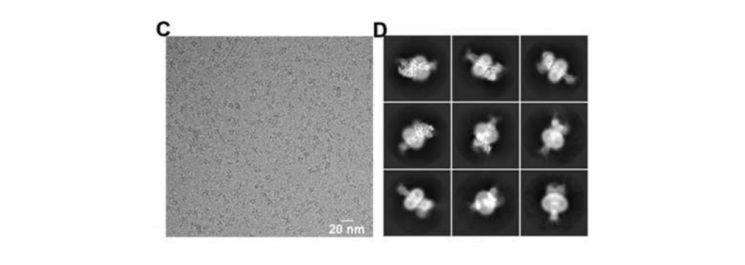
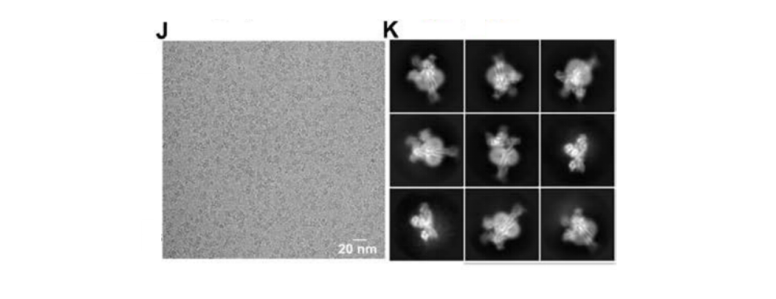
study, the structures of GCGR with its cognate ligand glucagon and heterotrimeric Gs or Gi1
protein were resolved by cryo-EM SPA to investigate the molecular mechanism of GCGR interaction
with G proteins.
-
+86 18962587269
-
contact@readcrystal.com
-
Changshu High-tech Industrial Development Zone, Suzhou, Jiangsu Province


 02 Feb
02 Feb