The Importance of Cocrystal Structures in Rational Drug Design
Rational Drug Design is a structure-based drug design approach. By studying the interactions between drug structures and in vivo targets, drugs can achieve desired objectives such as inhibiting enzyme activity, promoting the release of certain substances, or obstructing channels. This process largely depends on the understanding of the three-dimensional structures of targets and drugs, thus structural biology has had a profound impact on drug research. For the design of small molecule drugs, the cocrystal structure of the target protein-small molecule ligand (hereinafter referred to as "cocrystal structure") is the most critical information in the drug design process.
Cocrystal structure information not only reveals the binding modes and bioactive conformations, discovers new binding pockets or allosteric sites but also enriches rational drug design approaches such as Structure-Based Drug Design (SBDD), Fragment-Based Drug Design (FBDD), Computer-Aided Drug Design (CADD), and AI-driven drug discovery.
For the analysis of crucial cocrystal structures in drug development, the greatly reduced crystal size requirement of MicroED offers numerous practical advantages:
1.Increasing the success rate of structure analysis for difficult-to-crystallize samples.
2.Significantly improving the success rate of obtaining cocrystal structures.
3.Enhancing the efficiency of research and development iterations.
4.Reducing protein consumption.
The following examples illustrate the technical advantages of MicroED in detail.
Advantages of MicroED Applications (I)
Increasing the Success Rate of Structure Analysis for Difficult-to-Crystallize Samples, Resolving Structures Unresolvable by XRD
Case 1: HIV-1 Protease
During the maturation of the HIV virus, HIV-1 protease cleaves the Gag protein, assembling a mature HIV virus. Therefore, a potential therapeutic strategy is to block the interaction between Gag and HIV-1 protease. The small molecule inhibitor bevirimat is designed to inhibit the activity of HIV-1 protease. However, due to the difficulty in obtaining sufficiently large crystal samples, the cocrystal structure of HIV-1-Gag-bevirimat could not be resolved using X-ray crystallography. Ultimately, the structure of the HIV-1-Gag-bevirimat cocrystal was resolved through MicroED. This structure revealed the electrostatic and hydrophobic interactions between bevirimat and the protein, providing a foundation for structure-based drug design and the development of more effective inhibitors.
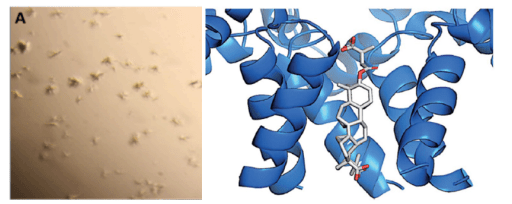
Fig 1. Structure of HIV-1-Gag-bevirimat resolved using MicroED technology [1]
Case 2: MicroED Resolves Membrane Protein Structures
The structural analysis of membrane proteins has always been a challenging problem for academia. The Lipidic Cubic Phases (LCP) technique can assist in the crystallization of membrane proteins, significantly advancing membrane protein crystallography. However, the crystals grown using LCP technology are often microcrystals, which are too small for XRD collection but are well-suited for MicroED technology. The image below shows the GPCR protein A2AR resolved using MicroED. A2AR is an important potential drug target, with studies showing its significant association with tumors, Parkinson's disease, drug addiction, and mental illnesses.
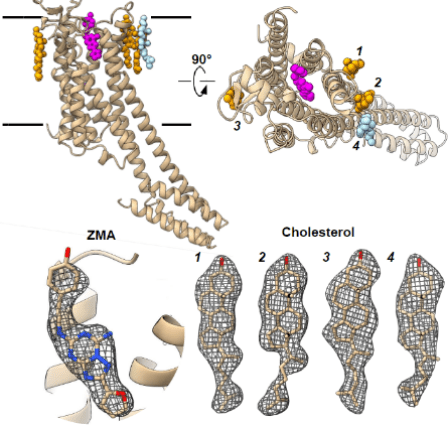
Fig 2. Structure of the membrane protein A2AR resolved using MicroED technology [2]
Case 3: MicroED Determines Peptide Structures
Peptides have important biological functions, and resolving their structures is crucial for understanding their physiological mechanisms. However, some peptide structures cannot be resolved using X-ray crystallography. For example, tau protein aggregation is associated with various neurological diseases (including Alzheimer's disease). The VQIINK segment of the tau protein drives the aggregation and formation of amyloid fibrils in the brain, so inhibiting this segment might halt disease progression. Researchers tried to grow large crystals of this segment but could only obtain microcrystals, making X-ray methods unsuitable for resolving this segment's crystal structure. Ultimately, MicroED technology was used to resolve the 1.1 Å resolution structure of the KVQIINKKLD segment. Based on the resolved structure, researchers designed a series of inhibitors and identified one with excellent inhibitory effects, as shown in the image below.
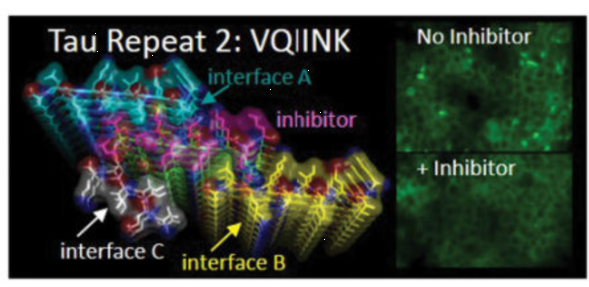
Fig 3. The inhibitor designed based on the VQIINK structure shows significant efficacy [3]
Advantages of MicroED Applications (II)
Significantly Improving the Success Rate of Small Molecule Cocrystals with Target Proteins
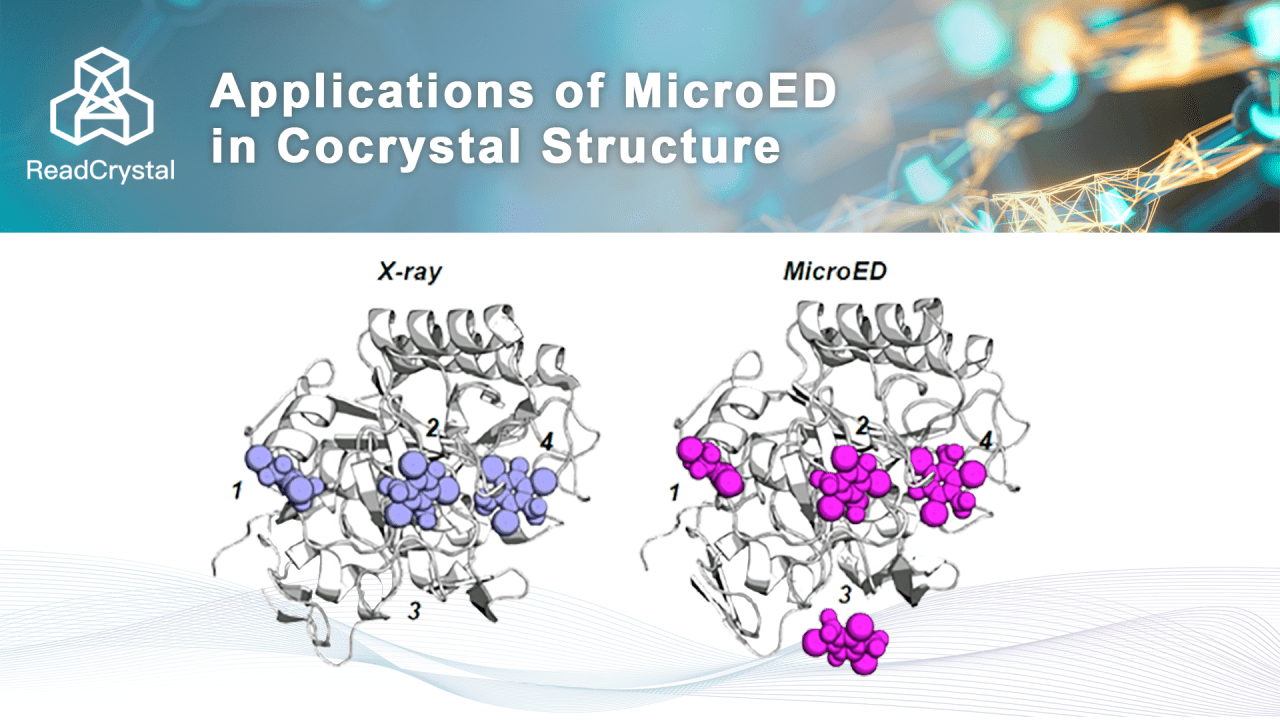
A common method for obtaining small molecule cocrystals with proteins is through soaking. Since X-ray crystallography requires sufficiently large protein crystals for structure analysis, the soaking process often causes large protein crystals to break, resulting in a decrease in crystal quality and failure to obtain cocrystal structures. MicroED requires only 100-nanometer protein crystals, which greatly reduces the likelihood of breakage during small molecule soaking and increases the spatial occupancy of small molecules. Therefore, MicroED can significantly improve the soaking success rate and the success rate of cocrystal structure analysis.
Researchers compared the differences between MicroED and X-ray crystallography in obtaining protein-small molecule cocrystals using 4 binding pockets of proteinase K soaked with the small molecule I3C. The results showed that, due to the nanoscale microcrystals used in MicroED, the efficiency of small molecules diffusing into protein pockets was very high. The occupancy rates of small molecules in all four binding pockets were better than those resolved by X-ray crystallography, as shown in the figure below. Therefore, the soaking effect of small molecules in microcrystals using MicroED is significantly better than that required for large crystals in X-ray crystallography. This will greatly accelerate the progress of FBDD, CADD, and AI drug development.
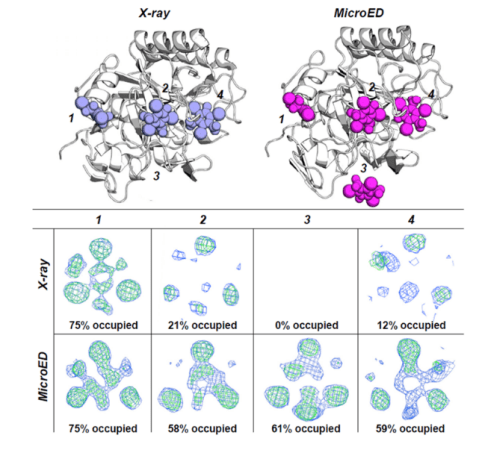
Fig 4. Comparison of small molecule occupancy in the protein binding pocket [4]
Advantages of MicroED Applications (III)
Enhancing R&D Iteration Efficiency
1.XRD requires large, perfect crystals. Often, it is difficult to obtain large crystals, or the large crystals obtained have many defects, requiring multiple rounds of optimization to improve crystal quality. In contrast, the nanocrystals needed for MicroED are easier to obtain, often have fewer defects, and are of higher quality, thus reducing the time needed for extensive optimization.
2.Laboratory MicroED equipment vs. scarce synchrotron beam time. MicroED equipment can be deployed in laboratories, allowing testing as soon as samples are available, sometimes on the same day. Synchrotron facilities, on the other hand, are national large-scale scientific devices, offering beam time only once every few weeks or months. Thus, prepared samples often have to wait a long time for beam time.
Advantages of MicroED Applications (IV)
Reducing Protein Consumption
Traditional XRD requires hundreds to thousands of crystallization screenings for protein structure analysis, resulting in high protein consumption. For some low-expression proteins, the cost of protein production is significant. In contrast, MicroED, due to its low crystal size requirement, low perfection requirement, and high soaking success rate, significantly reduces the number of crystallization screenings needed, thereby also reducing the consumption of recombinant proteins.
References:
[1]. PURDY M D, SHI D, CHRUSTOWICZ J, et al. MicroED structures of HIV-1 Gag CTD-SP1 reveal binding interactions with the maturation inhibitor bevirimat [J]. Proc Natl Acad Sci U S A, 2018, 115(52): 13258-63.
[2]. MICHAEL W. MARTYNOWYCZ A S, ET AL. MicroED structure of the human adenosine receptor 2 determined from a single nanocrystal in LCP [J]. biorix, 2020,
[3]. SEIDLER P M, BOYER D R, RODRIGUEZ J A, et al. Structure-based inhibitors of tau aggregation [J]. Nature chemistry, 2018, 10(2): 170-6.
[4]. MARTYNOWYCZ M W, GONEN T. Ligand Incorporation into Protein Microcrystals for MicroED by On-Grid Soaking [J]. Structure, 2021, 29(1): 88-95 e2.




