BTK protein is a member of the TEC family of non-receptor tyrosine kinases in the cytoplasm and plays a crucial role in the growth, development, proliferation, and differentiation of B cells. It is also a therapeutic target for B-cell malignancies.
ReadCrystal Biotechnology has completed the expression and structural analysis of the BTK target protein. Based on these experimental conditions and the available protein inventory, ReadCrystal has shortened the turnaround time to 1-2 months and ensures the successful delivery of the project.
Mutations in the gene encoding Bruton’s tyrosine kinase (BTK) lead to impaired B-cell differentiation and maturation, preventing the transition from pre-B cells to immature B cells. This results in a deficiency or significant reduction of mature B cells in peripheral blood and a marked decrease in the levels of various immunoglobulin isotypes in serum. This condition causes X-linked agammaglobulinemia (XLA), a primary immunodeficiency disease. The disease was first discovered by Dr. Bruton in 1952, and in honor of him, the mutated kinase was named Bruton’s tyrosine kinase, abbreviated as BTK.
The BTK protein (UniprotID Q06187) mainly comprises four parts: the PH and Tec domains, the SH3 domain, the SH2 domain, and the Kinase domain. The PH domain includes binding sites for the transcription factor BAP-135/TFII-I, the negative regulator PIN1, and IBTK, and is also responsible for mediating the interaction between BTK and the second messenger phosphatidylinositol trisphosphate (PIP3). The Tec domain is adjacent to the PH domain and includes the BTK motif (Zn cofactor binding site), the PKC-β binding site, and a conserved region rich in proline motifs. The Kinase domain contains the activation loop, ATP binding site, catalytic core, and allosteric inhibitory segment. BTK activation (phosphorylation) initially occurs in the activation loop within the Kinase domain (which is also the target region for small molecule inhibitors), with further activation occurring in the SH2 and SH3 domains, which contain the primary autophosphorylation sites. These SH domains also contain the nuclear localization signals (NLS) and nuclear export sequences (NES) necessary for BTK nuclear-cytoplasmic shuttling.
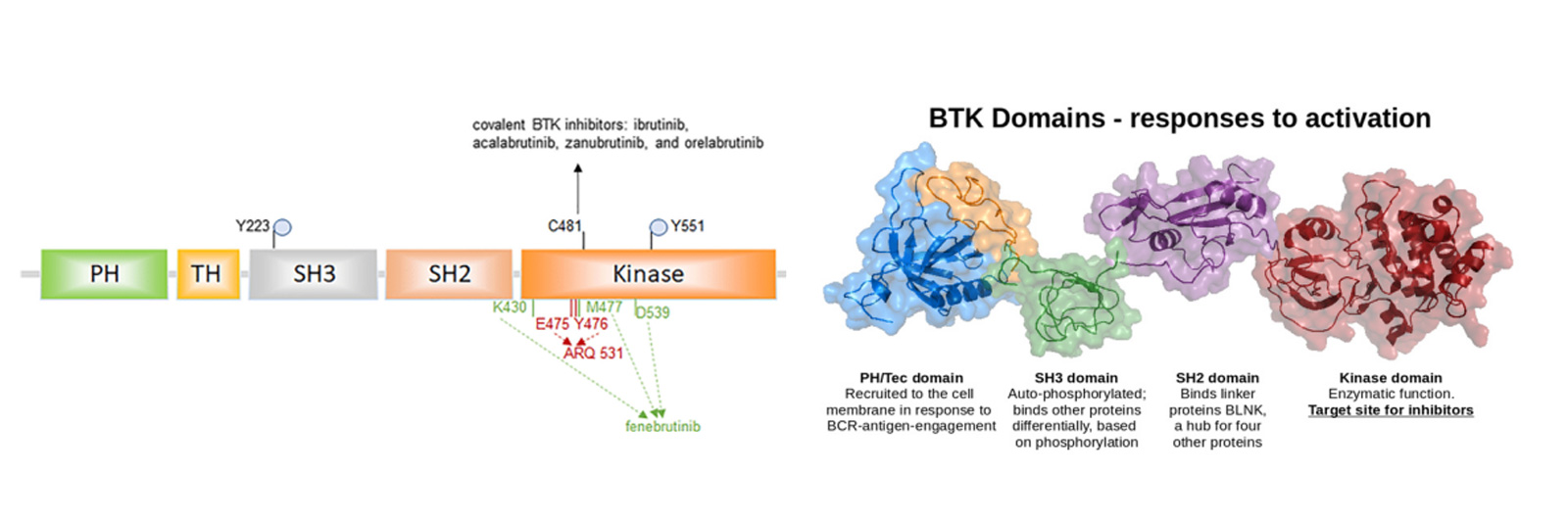
Abnormalities in BTK are closely related to cancer and autoimmune diseases. On one hand, the BCR pathway is highly active in malignant B cells, leading to abnormal proliferation of B cells and contributing to the development and progression of malignancies such as non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL). Studies have shown that BTK is often overexpressed in CLL, accompanied by an increase in its phosphorylation levels. Research using CLL mouse models has also found that BTK-deficient mice do not develop CLL, while mice with BTK overexpression exhibit significantly higher rates of CLL incidence and mortality. BTK plays a critical role in the growth, migration, and other processes of B-cell malignancies, making it an excellent target for the targeted therapy of these cancers. On the other hand, BTK is also essential in signaling within myeloid cells, and microglia in the central nervous system have been shown to express high levels of BTK in multiple sclerosis.
About ReadCrystal
ReadCrystal has successfully completed the expression, purification, and crystallization of the BTK protein, as well as accumulated rich data on the co-crystal structures of this target and its inhibitors. We also has extensive experience with proteins related to the BTK pathway, which can shorten the turnaround time to 1-2 months and ensure successful delivery, meeting the drug development needs of enterprises.

BTK Protein Experimental Results
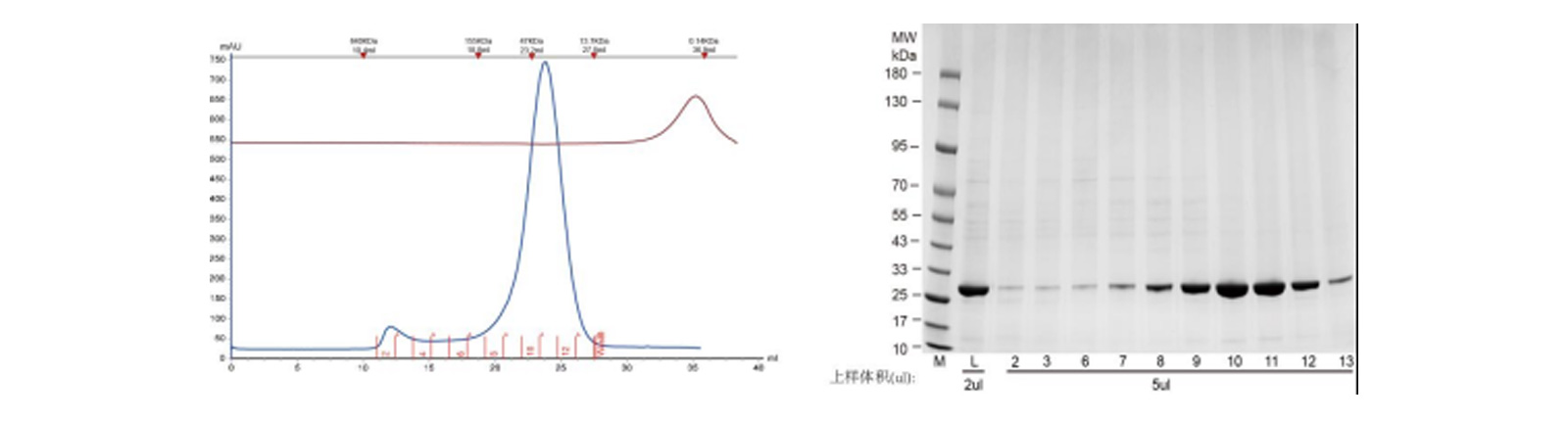
Expression & Purification Results (Turnaound Time: in-stock 1 week, custom 1 month)
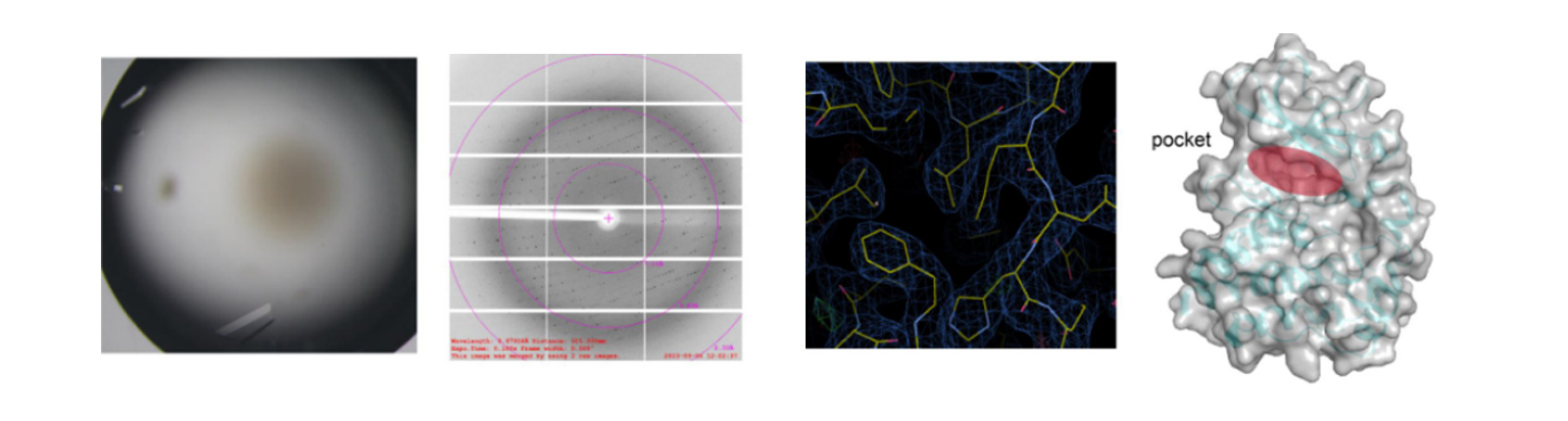
Crystal Diffraction & Structural Analysis (Turnaound Time: 2-4 weeks)